Cite as: Benner, S. A. (2021) “An Earth-Sized Planet 41 Light Years Away with Hydrogen Cyanide. So What?”. Primordial Scoop, 2021, e0328. https://doi.org/10.52400/YFDI7298
A team at Jet Propulsion Laboratory just reported a study of the atmosphere a rocky planet (GJ 1132) orbiting a red dwarf star 41 light years away from US. The SciTechDaily report remarked that the atmosphere is smoggy, hazy, and “contains a toxic mix of hydrogen, methane, and hydrogen cyanide.”
All OK. Except the word “toxic”.
Well yes, hydrogen cyanide is toxic to us, Homo sapiens. But hydrogen cyanide was also likely involved in the origin of life. So let us say a few words about this remarkable molecule, built from just one hydrogen atom (H), one carbon atom (C), and one nitrogen atom (N). That is H1C1N1, or HCN. A single bond holds the hydrogen to the carbon, and a triple bond holds the carbon to the nitrogen.
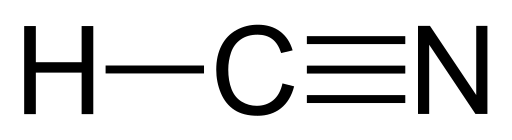
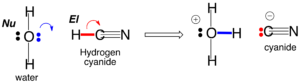
Chemistry has two concepts that are horribly misnamed: “nucleophile” and “electrophile”. “Nucleophiles” do not “love nuclei”, “Electrophiles” do not “love electrons”, and students are perpetually confused by teachers do say that they do.
The correct way to understand nucleophiles and electrophiles is to put them together in a chemical reaction where they form a new bond. That new bond requires that a pair of electrons come from somewhere. The nucleophile is the somewhere; it is the reactant that brings the pair of electrons. The electrophile is the reactant that does not.
What is special about HCN? Well, consider the one carbon atom and how it reacts with a nucleophilic center such as the oxygen atom on water. That oxygen has two pairs of electrons. One of those pairs can form a new bond to the carbon atom of HCN.
Since the carbon atom already has four bonds, the maximum for carbon, one of the bonds in the C-N triple bond must break for the new oxygen-carbon bond to form. The now-broken bond is a free pair of electrons that goes onto the nitrogen, which makes the nitrogen a nucleophilic center. Its electrons can form a new bond to an electrophilic center. If that electrophilic center is a hydrogen on water, a new nitrogen-hydrogen bond is formed. This is the first step in the hydrolysis of HCN, a reaction that has a half-life of approximately 10 years at room temperature. Have a look.

But that’s not the only reaction that HCN can do. For example, one of the pairs of electrons on the oxygen atom of water can form a new bond to the hydrogen atom of HCN. Since the hydrogen atom already has one bond, the maximum for hydrogen, the bond holding the hydrogen atom to the carbon atom must break. That frees up a pair of electrons that ends up on the carbon atom.
The product of a reaction where HCN loses H is simply called cyanide. But unlike hydrogen cyanide, where the carbon atom entered the previous reaction as an electrophile, here, the carbon atom of cyanide has a pair of electrons available to form a new bond. Therefore, this carbon atom of cyanide is now a nucleophile.
So get this. HCN has a carbon atom that can react under some conditions as either an electrophile or a nucleophile. Indeed, the carbon atom on one hydrogen cyanide can react as an electrophile with the carbon atom of cyanide (CN). Cyanide delivers the electron pair to form a new carbon-carbon bond in a product that has the formula H2C2N2. Have a look.
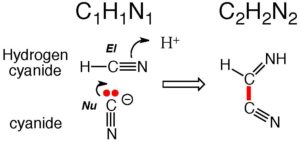
It is unusual in organic chemistry for a carbon atom in a molecule to be able to behave sometimes as a nucleophile and sometimes as an electrophile. HCN is one of those unusual molecules. Therefore, HCN can do things that most other molecules cannot do, that is self assemble themselves to give higher molecular weight species with many carbon-carbon bonds. For example, we can react cyanide as a nucleophile with the H2C2N2 that we just formed to give a bigger product, H3C3N3. And if we add two more H1C1N1‘s as electrophiles, we get H5C5N5. And this is not just any H5C5N5. Rather, it is adenine, one of the building blocks of RNA, thought by some to be the first molecule to support Darwinian evolution on Earth.
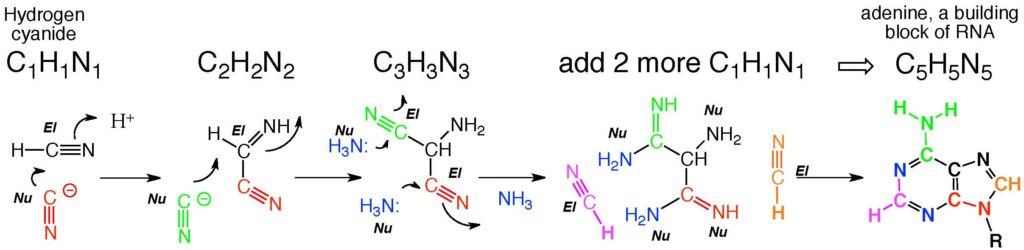
So think about the report from JPL in a slightly different light. Just 41 light years from Earth there is a planet with the ability to form adenine, one of the building blocks of life.