Cite as: Benner, S. A. (2021) “Benzoic acid on Mars. A 20 year old prediction come true?”. Primordial Scoop, 2021, e1104. https://doi.org/10.52400/AZXH5253
Since its arrival on Mars in 2012, NASA’s Curiosity rover has been puttering around Gale Crater looking for signs of ancient habitable environments. Not looking for extant life, of course. Because of NASA’s institutional cowardice, discussed elsewhere on this blog, NASA cannot do that.
So looking for organic molecules will have to do.
Curiosity has already found evidence for Martian organics. They were released from drilled rock samples by heating (~850 °C). Volatilized gases were then analyzed by gas chromatographic (GC) separation followed by mass spectrometry (MS). As discussed elsewhere on these pages, this analysis can run afoul of any perchlorate in the sample; perchlorate at these temperatures burns organics.
Nevertheless, the team manning the the Sample Analysis at Mars (SAM) platform also reported fragments of alkyl and aromatic compounds in sedimentary rocks on Mars, as well as sulfur-containing organics and some chlorinated hydrocarbons, likely products of perchlorate-mediated combustion.
But the SAM platform also can add silylating reagents to samples. In many cases, this will make otherwise non-volatile organics fly in a GC-MS.
As Millan et al. tell the story in their groundbreaking publication, a sample had been gotten on the Bagnold Dunes (of Mars, not III Delta Kaising). However, the sample was not immediately examined for organics. The team thought that the dunes were exposed to ionizing radiation that was presumed to destroy organics, and therefore gave this sample processing lower priority. Therefore, it required the drill to be out of commission for the dune sample to be silylated.
And when it was, Millan et al. found benzoic acid, in its silylated form. A benzene ring with a -COOH carboxyl group attached.
A molecule that we and our coworkers predicted, 20 years ago, would be found.
The experimental analysis is not trivial, as the silylating reagent itself gives background signals. Also, species (like borate) that would, absent contamination, be extremely interesting to detect, are extracted from materials (like glass) used in the instrument. In this case, Tenax, which is used in the instrument’s trap, has a 2,6-diphenyl-p-phenylene oxo polymer unit; this can be oxidized to give benzoic acid. Millan et al. considered all of these, and they appear have done the calculation correctly to rule those contaminants out.
So perhaps the report of benzoic acid is real.
Why did we predict 20 years ago that benzoic acid would be present?
First, we knew in 1999 that organic molecules come to Mars by meteoritic infall. And we have samples of those organics, because the same processes delivers them to Earth. And the organics land on the dunes of Mars, just like they land on the dunes of the Sahara Desert, or the dunes of III Delta Kaising (what part of the word “fiction” do I not understand?).
Now, much of those organics is carbon on the way to forming graphite, a network of carbon atoms that form six-membered rings. These 6-membered rings are called “benzene” or “aromatic” rings. Indeed, many of these benzene ring compounds do have spice-like aromas.
Further, we also knew in 1999 that the surface of Mars is mildly oxidizing, if only because of the photolysis of water followed by the gravitational escape of dihydrogen, leaving behind hydrogen peroxide.
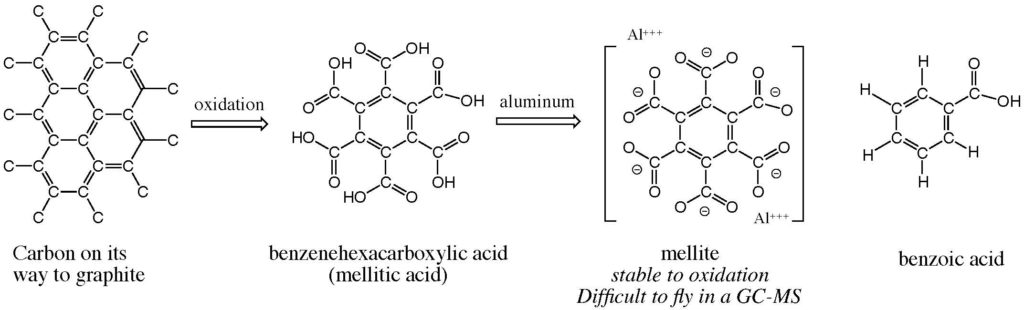
We knew that the generic oxidation of condensed aromatic rings burned such species down to a core benzene ring to which carboxylic acid groups (-COOH) were attached. The greater the number of attached carboxyl groups, the more stable the benzene ring becomes to further oxidation.
This process is known on Earth. Thus, coal is carbon on its way to graphite. The terran atmosphere is mildly oxidizing. And coal exposed to the atmosphere is slowly converted to benzene carboxylates. Some of these are even minerals. For example, the aluminum salt of benzene with six carboxylate groups is the mineral mellite, named because of its honey-like color (mel- is an Indo-European root for “honey”). The featured image shows well this sweet color.
Our hypothesis 20 years ago added to our understanding why the Viking 1976 mission to Mars (likely) detected the signs of a Martian biosphere, at least under the terms of the experiments themselves. The data meet all of the requirements for affirmative life detection proposed by Neveu et al. a few years back.
Those affirmative life detection experiments were dismissed at the time (and even today by NASA committees lacking the needed understanding of organic chemistry) because a GC-MS experiment did not detect organic samples at the two sites where the Viking dual mission landed. We now know that this failure was due to multiple issues, including instrument insensitivity, the aforementioned perchlorate, and the fact that mellite and similar molecules are not easily volatilized to enter a GC-MS.
So where is spice-like Martian mellite? Well, it is probably still sitting there in whatever samples Curiosity still has from the dune. Even silylation does not allow mellitic acid to fly, especially as an aluminum salt (which is the organic mineral mellite).
This has implications for the search for life on Mars. As Jan Špaček discussed on these pages earlier this year, Martian life is likely to be found in layers of life and dust in the Martian permafrost. The amount of energy available to support that life is likely to be less than energies available in still hypothetical geothermal sites. If meteoritic organics can survive in the dust clouds that circle Mars and deliver living organisms from those sites to the dunes, perhaps so can Martian bio-organics.
It is time to stop looking for “habilitability” and flying toy drones around Mars. It is time to look for life on Mars. We know how to look. We know where to look. When we have looked before, we found evidence for life on Mars. Before humans contaminate the surface, the search for life on Mars must start now.
And if institutional cowardice means that NASA will not do it, some other organization needs to come forward to do it.